the phase change behavior of sodium acetate trihydrate
Sodium acetate is mainly known for its use in heating pads, hand warmers, and hot ice. Sodium acetate trihydrate crystals melt at 58 °C. When they are heated beyond their melting point and subsequently allowed to cool, the aqueous solution becomes supersaturated. This solution is capable of cooling to room temperature without forming crystals. By agitating the solution it crystallizes back into solid sodium acetate trihydrate. The bond-forming process of crystallization is exothermic. The process can be repeated indefinitely.
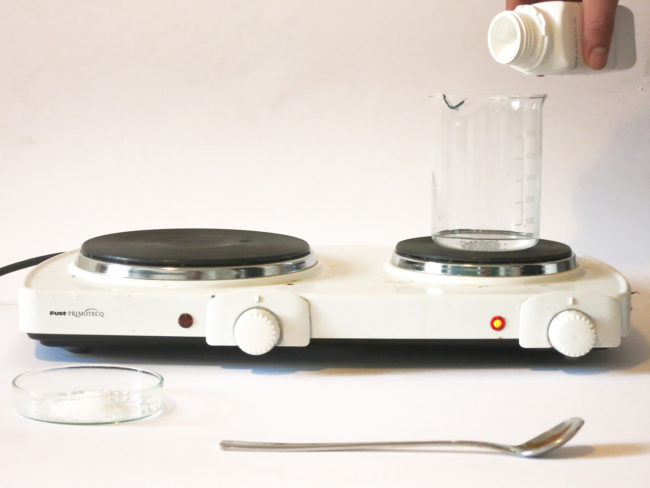
Materials and Tools
|
|
1. Prepare Supersaturated Solution
Pour the water into the pot or glass beaker. Add the sodium acetate trihydrate powder while continuously stirring the mixture. Heat it gently and stir until all crystals of sodium acetate have dissolved. You can also heat the solution in the microwave for 1-2 minutes until boiling. Continue heating and stirring until the solution has turned clear.
2. Cooling
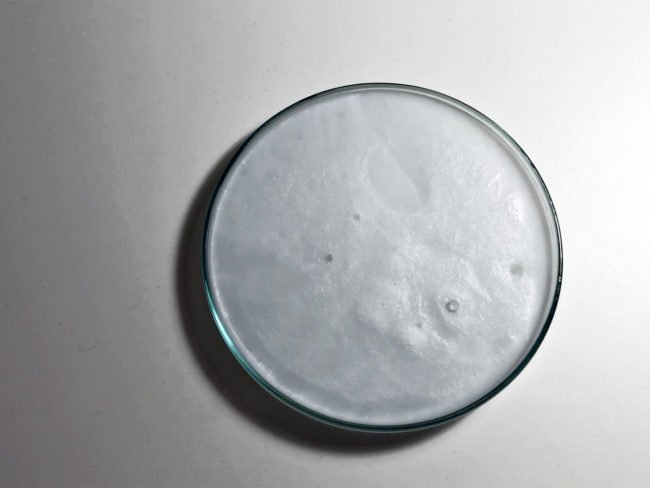
Remove the flask from the heat and let it cool slowly without disturbing it. This may take up to several hours. To speed up the process the solution can be placed into a fridge. When the solution is ready it turns milky.
3. Activate
In order to enhance the visual effect in this case thermochromic (color-changing) pigments have been added to the solution. Since leuco-dye pigments however are not water-soluble it didn’t work as well as expected. Dissolving the pigments in alcohol did the trick but when adding it to the sodium acetate solution the mixture got corrupted and didn’t work as properly as before.
The solution can be activated by various means. One possibility is to drop a single crystal of sodium acetate. Another possibility is through mechanical force. Once activated the solution rapidly crystallises and gives off heat, which in this case causes the color to change from blue to white.
4. Repeat
The process can be repeated indefinitely. Simply reheat the crystallised solution until it turns liquid again.